Guidelines regarding the use of AI/ML in healthcare are constantly evolving, with massive implications for training data generation, annotation, and validation.
Regulatory Compliance
High-quality data annotation for verifying and validating AI-based medical devices to achieve regulatory approval from national and international regulatory bodies

Do your data annotation processes combine the necessary expertise and knowledge of regulatory standards?
Are you equipped to overcome regulatory hurdles to ensure a faster time-to-market for your products?
Medical Data Annotation by iMerit
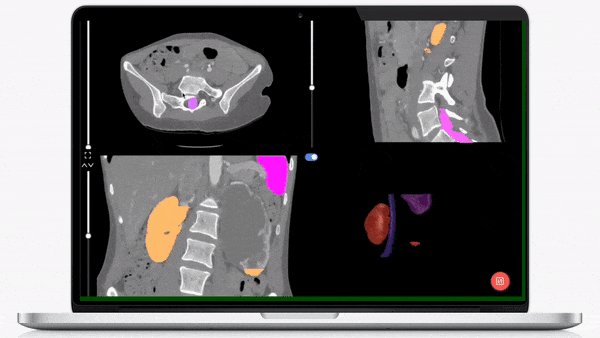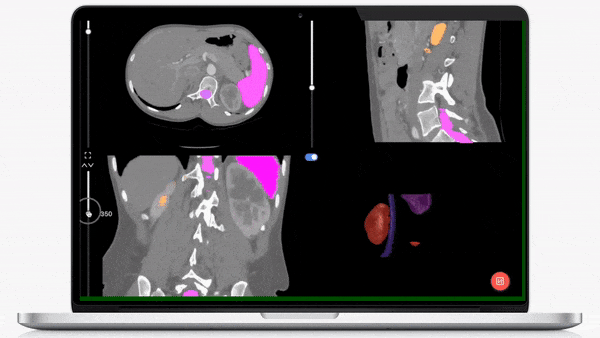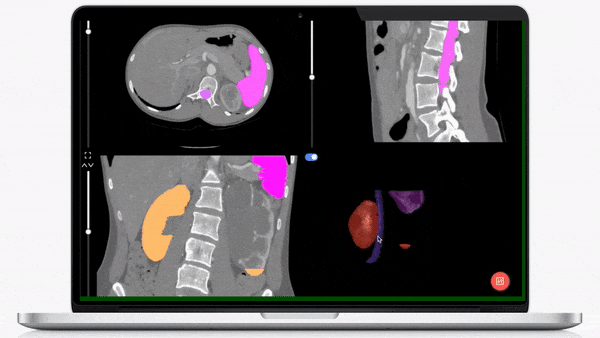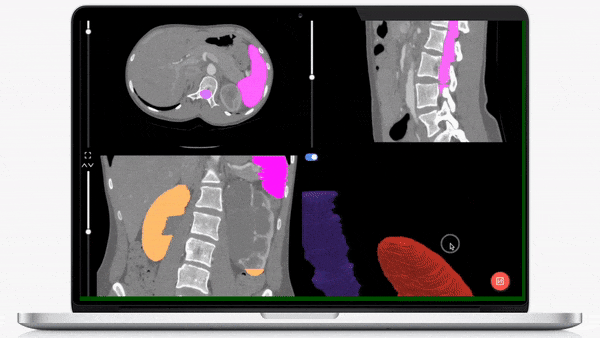
iMerit’s physician-led teams specialize in creating regulatory-grade training data pipelines and operations that help achieve regulatory validation for your AI-powered product launch.
- iMerit’s toolset integrates CFR 21 part 11 and Annex 11 regulatory tracking into workflows, ensuring all annotations are up to the standards of evolving regulations.
- Throughout the ML journey, iMerit experts evaluate laws and legal proceedings to anticipate or accommodate new precedents.
- All workflows leverage iMerit’s extensive tool ecosystem and accommodate rapidly evolving HIPAA and FDA guidelines.
- Our teams and tools are trained in and comply with all relevant GxP processes for medical device and clinical trials.
High-Quality Training Data
iMerit supports AI/ML teams in the healthcare industry by combining FDA-compliant training data pipelines with critical medical expertise.
- Specialized medical annotators work hand-in-hand with doctors in hybrid workflows
- US board-certified physicians perform benchmarking and validation
- HIPAA-compliant annotation processes will ensure data stays protected and secure
- Our regulatory-grade processes ensure AI-assisted products obtain FDA 510(k) clearance
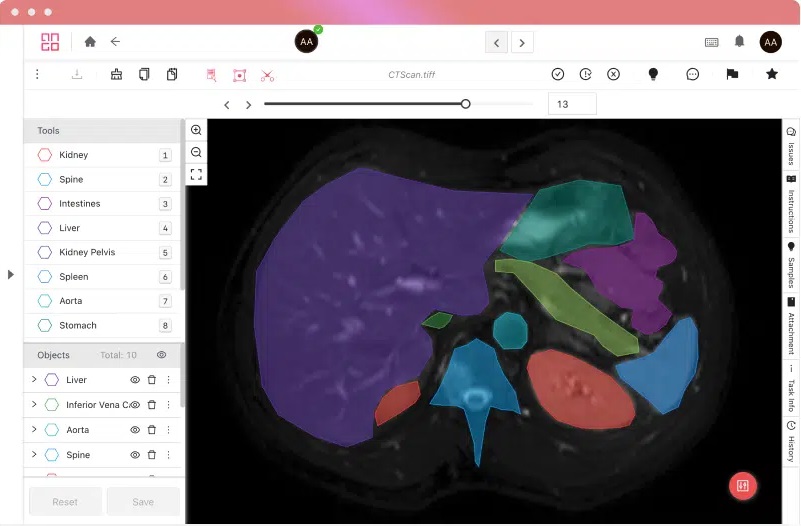
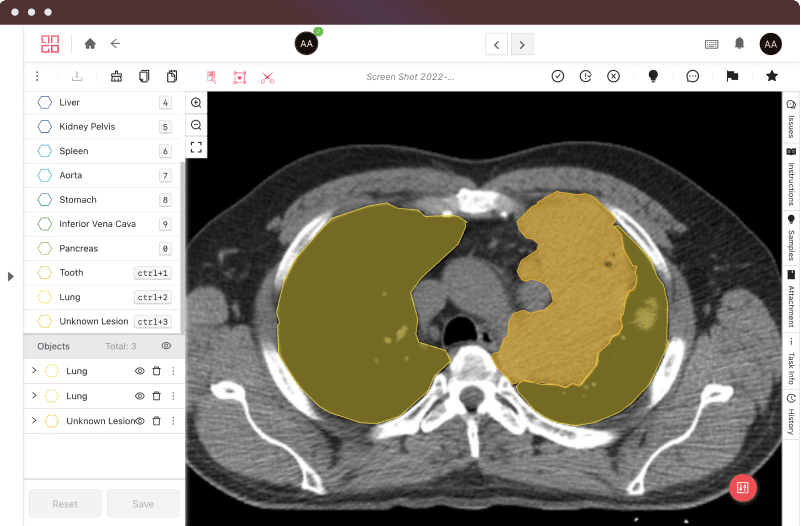
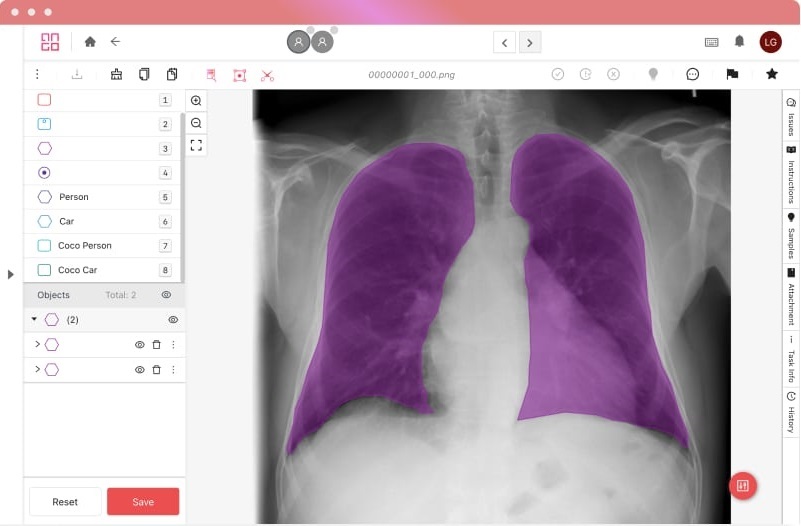
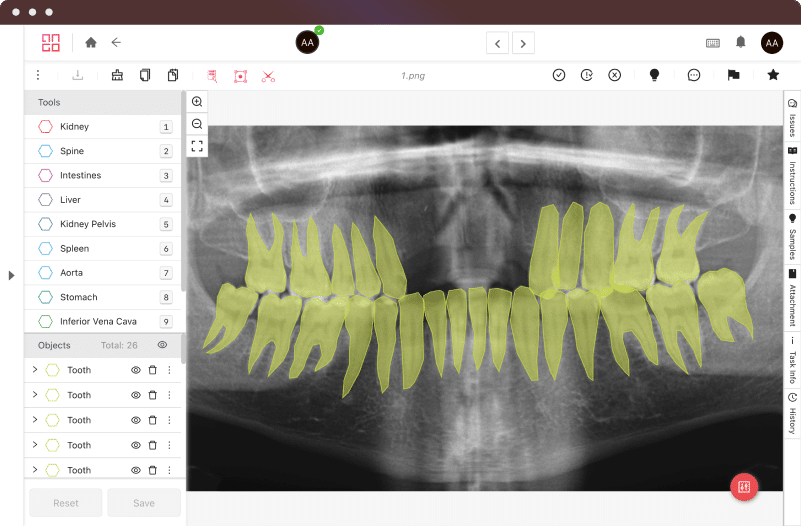
Certifications
iMerit is ISO 27001:2013 certified, SOC 2 compliant, GDPR certified, AICPA SOC certified, CFR 21 Part 11, and HIPAA compliant. We undergo regular audits and reviews to maintain these certifications. During these audits, our internal controls for security, availability, confidentiality, privacy, and processing integrity are checked rigorously through documentation reviews and onsite visits.
Most Trusted Platform for Training Data
Our in-house data annotation tools ensure your data is secure, private, and protected. With strict access control, proper data partitioning, and encryption of sensitive information, our tools support and adhere to data privacy regulations and compliance standards. Detailed logging and auditing capabilities help track access and actions performed on the data, which is essential for security monitoring.
HIPAA
With iMerit, all data annotation is certified HIPAA compliant and all information is physically and digitally safeguarded.
EU Annex 11
iMerit consults on training data generation, validation, and deployment to ensure automation does not lead to lower quality, service, or control.
FDA 510(k)
iMerit’s personnel and tools stay ahead of FDA requirements to make sure your products are launched on time.
CFR 21 Part 11
iMerit’s proprietary tools and functionalities ensure compliance with CFR 21 Part 11 digital signatures.
GxP
iMerit’s teams are trained on Clinical Data Management, Data Integrity Awareness, and Data Governance in support of GxP Clinical Trial standards.
Case Study

Leading MedTech Startup Partners with iMerit for Regulatory-grade Data Sourcing
To transform the diagnosis and treatment of degenerative arthritis, this company needed to source radiological imagery for its computer vision model.
Using iMerit-sourced and annotated radiological imagery, their computer vision model achieved successful FDA 510(k) approval while reducing project cost by 72%.
99
%
Annotation Accuracy
510
K
FDA Approval
72
%
Cost Reduction
"We needed a one-stop shop for data sourcing, labeling, and validation. We needed guarantees, not more uncertainty. "
Chief Technology Officer, MedTech Startup


CONTACT US
