Regulatory Validation for Arthritis
Diagnosis and Treatment
FDA
Approval Granted
This well-funded startup relies on iMerit for regulatory-grade data sourcing, HIPAA-compliant data management, expert 3D radiological segmentation, and FDA benchmarking.
Challenge
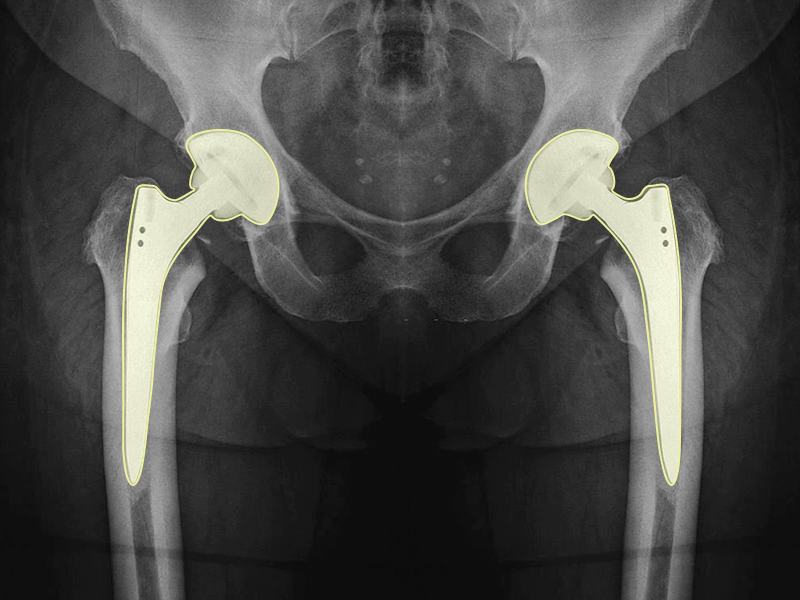
Using computer vision, this MedTech startup transformed the diagnosis and treatment of degenerative arthritis. As this company shifted focus to manufacturing custom joint replacements, they needed to source 3D radiological MRI and CT scans of the hip. This imaging would also require 3D segmentation at a scale beyond what their internal resources could normally accomplish fast and affordably.

As their in-house teams sourced radiological imagery, each new source of data was creating project delays. The teams were constantly re-evaluating evolving FDA guidelines, which demand certain criteria be met around data diversity and validation. This, combined with the time necessary for voxel-perfect manual segmentation meant their project would be critically delayed.
“We needed a one-stop shop for data sourcing, labeling, and validation. We needed guarantees, not more uncertainty.”
- CTO
Solution
Upon meeting this company, iMerit’s data curation teams went to work sourcing the necessary CT and MRI scans from its network of providers. Segmentation tasks were migrated to iMerit’s Radiology Suite so that data could be labeled at scale on a secure platform. Custom workflow features allowed for cost efficient use of Radiologist and second-level time through escalations and edge case routing.
A total of 5,000 Pelvic CTs and 5,000 Pelvic MRIs of degenerative arthritis and fracture were sourced and segmented. To optimize annotation costs, iMerit recommended a hybrid workflow model, where specialized medical annotators would perform the bulk of the annotations while India-based Radiologists would perform the quality checks.
Once completed, the diagnostic model needed to undergo validation for FDA regulatory approval. To accomplish this, iMerit assembled a team of US-board certified orthopedists, radiology generalists, and musculoskeletal-fellowship trained radiologists to perform create consensus ground truth and benchmarks against a validation dataset that was balanced by gender, race, acquisition protocol, and geography.
“iMerit sourced the images, labeled it scalably, guided us towards the critical validation we needed, and helped us launch on time.”
- CTO
Result
By keeping up with a rapidly-evolving FDA landscape, iMerit was able to guide this startup to successful FDA approval. iMerit’s proprietary toolset, programmatic record keeping, and electronic signature was used to fulfill FDA CFR 21 part 11 requirements. Quality benchmarking against a gold-set dataset showed iMerit’s hybrid workflow achieved 99% annotation quality, resulting in a substantial 98.8% arthritis diagnostic accuracy.
Thanks to iMerit, this company met their critical launch window. The company performed a cost analysis and found iMerit’s hybrid workflow enabled them to reduce project costs by 72%. Today, iMerit continues to function as an extension to this company’s in-house teams, helping with data sourcing, annotation overflow, and consulting on evolving regulatory challenges.