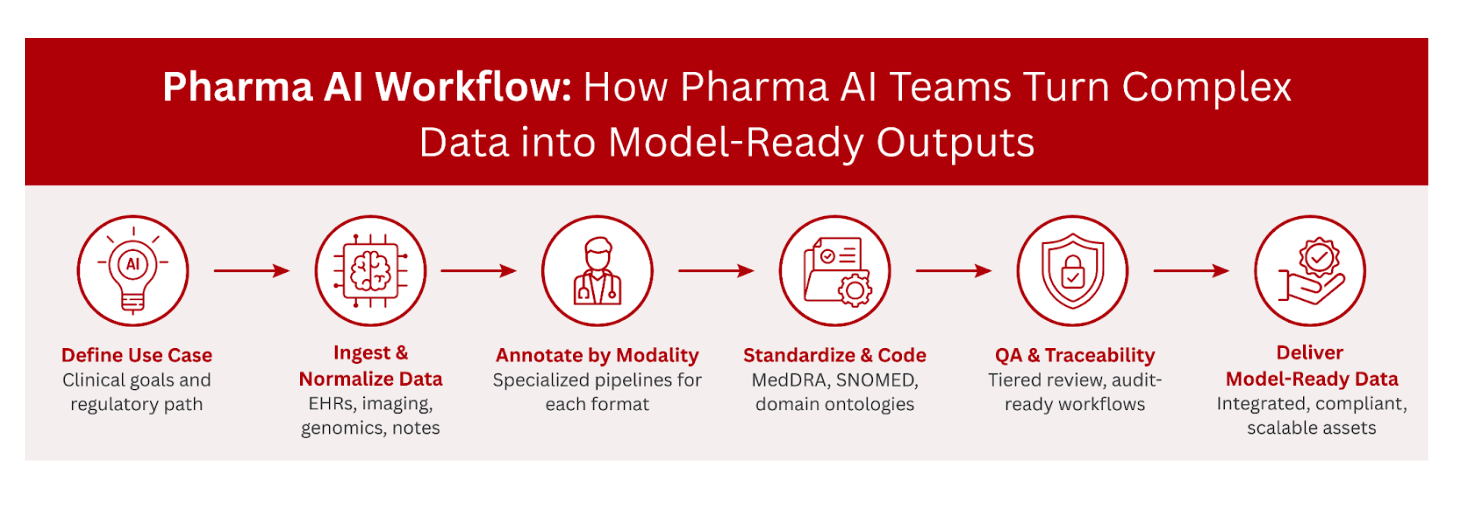
AI is no longer experimental in pharma. It is embedded into the R&D pipeline, supporting everything from biomarker discovery to real-world evidence (RWE) and clinical trial optimization. But building clinical-grade AI takes more than raw data. It requires annotation workflows that are accurate, traceable, and aligned with regulatory and governance standards.
Despite growing adoption, pharma teams face real bottlenecks when turning complex healthcare data into reliable training sets. Fragmented records, lack of standardization, and modality-specific silos often stand in the way of model performance and regulatory trust. iMerit partners with clients to overcome these obstacles through precise annotation workflows, domain expertise, and infrastructure built for clinical reliability.
Harmonizing Longitudinal Clinical Records for Real-World Evidence
One of the most common issues we encounter in RWE projects is fragmented patient data. Clinical notes, imaging reports, lab values, and prescriptions often exist in disconnected formats across time. For AI models to make sense of disease progression or treatment response, these records must be harmonized with clinical precision.
We addressed this by building a structured annotation workflow that links events over time for our clients. Annotators were trained to extract timestamps, outcomes, and interventions while aligning them with clinical milestones. Our team implemented a date-based labeling strategy using standard ontologies like SNOMED and MedDRA. As a result, our client’s RWE models were able to track patient journeys more accurately and generate insights that held up to regulatory scrutiny.
Our Remote Patient Monitoring case study highlights how iMerit structured and annotated longitudinal data from various modalities to create coherent patient journeys.
Encoding Adverse Drug Reactions from Free Text
Capturing adverse drug reactions (ADRs) from clinical documents is another challenge we help clients solve. ADRs are often buried in unstructured narratives and described in varying formats. For pharmacovigilance AI to be effective, this information must be consistently mapped to MedDRA codes with contextual detail.
Our solution combined AI-assisted pre-labeling with expert manual review. Annotators identified reaction terms, mapped them to standard codes, and added structured context such as severity, onset, and resolution. This multi-layered annotation process improved precision and reduced false positives in downstream models used for signal detection and regulatory monitoring.
Scaling Multimodal Annotation for Biomarker Discovery
In biomarker discovery initiatives, pharma companies increasingly rely on integrated datasets combining histopathology, genomics, and clinical documentation. Each modality requires its own annotation logic, but the output must ultimately align to a single model-ready format.
We solved this by creating an end-to-end multimodal pipeline on our Ango Hub platform. Our pathology experts annotate cellular structures on WSI slides while our clinical annotators label variant effects and add contextual factors from EHRs. These inputs are merged into a unified dataset with built-in traceability. With visualization tools and quality checks across all layers, we help the clients produce an integrated data asset suitable for high-impact biomarker modeling.
Why iMerit for Pharma AI
Pharma AI demands more than standard annotation. It requires deep therapeutic knowledge, workflow agility, and compliance-grade systems. At iMerit, our curated scholars workforce includes domain experts in oncology, radiology, immunology, neurology, pharmacovigilance, and other therapeutic areas. We support multimodal annotation across imaging, genomics, EHRs, and tabular data with infrastructure built for traceability and scalability.
Our Ango Hub platform enables expert-in-the-loop reviews, audit logs, and model-in-the-loop workflows. Combined with our dual-shore/offshore workforce models and certified data environments (GxP, HIPAA, SOC 2, ISO 27001), we deliver not only high-quality labels but also confidence in the results.
Let’s Build AI That Works in the Real World
Whether you are mapping patient journeys, labeling multi-modal clinical trial data, or building real-world evidence platforms, we are here to help. At iMerit, we help AI-first pharma teams transform complex, multimodal datasets into model-ready assets. With deep domain expertise, secure infrastructure, and intelligent tooling, we deliver data pipelines that scale with science and comply with regulations.
Schedule a demo to connect with our experts and explore our Pharma AI solutions.